WHAT CAUSES AN HAE ATTACK?
What is hereditary angioedema (HAE)?
HAE is a rare inherited disease that can cause attacks of swelling, and often pain, in specific parts of the body, including the abdomen, face, and throat.
HAE attacks are caused by low levels of functioning C1-esterase inhibitor (C1-INH), a key protein in the body that controls swelling and inflammation.
- People with HAE have a genetic disorder that results in either too little (type I) or nonfunctioning (type II) C1-INH, making them susceptible to sudden and unpredictable attacks of swelling.
- In very rare cases, a person can experience HAE attacks even though C1-INH levels and functionality appear normal.
THE SYMPTOMS OF AN HAE ATTACK
What happens during an HAE attack?
- During an attack, abdominal, facial, and laryngeal swelling often gets worse over a period of 12 hours to a day, then gradually resolves over the following 2–5 days.
- Unlike allergic reactions, there is no itching or redness with HAE.
- In addition to abdominal swelling and pain, people often experience nausea, which can be accompanied by vomiting.
- Attacks of HAE in the throat are the most dangerous because swelling that closes off the airway may be life threatening.
- Abdominal attacks cause acute pain in the abdomen, and sometimes also cause nausea, vomiting, and diarrhea.

Photo does not depict actual patient.
–BERINERT patient
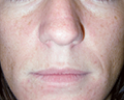
Normal
appearance
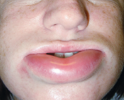
During cutaneous
attack: Nonpitting
edema

Normal
appearance

During
abdominal attack:
gastrointestinal pain,
nausea, vomiting,
swelling of abdomen
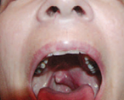
Normal
appearance
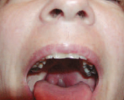
During laryngeal
attack: voice
change, hoarseness,
swelling of larynx
LIVING WITH HAE
HAE attacks typically begin in childhood, becoming more severe over time. The number of episodes an individual may experience is unpredictable. Some people have weekly attacks, while others may go years without one.
- HAE attacks can occur suddenly and without much warning. Worrying about your next attack can cause a great deal of stress for you and your family, but having a fast and reliable on-demand treatment plan can help put you back in control.
- Recognizing the early signs of an HAE attack can help you treat early.
Common warning signs that can signal onset of an HAE attack include:
- Sudden mood changes
- Irritability or aggressiveness
- Anxiety
- Extreme fatigue
- Rash or tingling sensation of the skin where the swelling begins
- Nausea
Recognize your triggers
Although HAE attacks are often unpredictable, some potential triggers have been identified:
References: 1. Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161(20):2417-2429. 2. Bork K, Meng G, Staubach P, et al. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):264-274.



